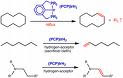
Dehydrogenation
Posted by
Chemical GoBlog
Labels:
#articel
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen (H2). It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures.
There are a variety of classes of dehydrogenations:
* Aromatization - Six-membered alicyclic rings can be aromatized in the presence of hydrogenation catalysts, the elements sulfur and selenium, or quinones (such as DDQ).
* Oxidation - The conversion of alcohols to ketones or aldehydes can be effected by metal catalysts such as copper chromite. In the Oppenauer oxidation, hydrogen is transferred from one alcohol to another to bring about the oxidation.
* Dehydrogenation of amines - amines can be converted to nitriles using a variety of reagents, such as IF5.
* Dehydrogenation of paraffins and olefins - paraffins like n-pentane and isopentane can be converted to pentene and isoprene.
Dehydrogenation converts saturated fats to unsaturated fats.
Enzymes that catalyze dehydrogenation are called dehydrogenases.
There are a variety of classes of dehydrogenations:
* Aromatization - Six-membered alicyclic rings can be aromatized in the presence of hydrogenation catalysts, the elements sulfur and selenium, or quinones (such as DDQ).
* Oxidation - The conversion of alcohols to ketones or aldehydes can be effected by metal catalysts such as copper chromite. In the Oppenauer oxidation, hydrogen is transferred from one alcohol to another to bring about the oxidation.
* Dehydrogenation of amines - amines can be converted to nitriles using a variety of reagents, such as IF5.
* Dehydrogenation of paraffins and olefins - paraffins like n-pentane and isopentane can be converted to pentene and isoprene.
Dehydrogenation converts saturated fats to unsaturated fats.
Enzymes that catalyze dehydrogenation are called dehydrogenases.