Fatty alcohol

Fatty alcohols are aliphatic alcohols derived from natural fats and oils, originating in plants, but also synthesized in animals and algae. Their significance in nutrition and health has historically been overlooked, and is only now being realized, as they are closely related to fatty acids, including the well-documented omega 3 fatty acids. The other counterparts are fatty aldehydes. Fatty alcohols usually have even number of carbon atoms. Production from fatty acids yields normal-chain alcohols—the alcohol group (-OH) attaches to the terminal carbon. Other processing can yield iso-alcohols—where the alcohol attaches to a carbon in the interior of the carbon chain.
Current and future uses
The smaller molecules are used in cosmetics and food, and as industrial solvents. Some of the larger molecules are simply seen as biofuels, but little research has been done until 2006 regarding many of these, and they have been shown to be have anticancer, antiviral, antifungal, anti-HIV properties, for potential use in medicine and health supplements.
Due to their amphipathic nature, fatty alcohols behave as nonionic surfactants. They find use as emulsifiers, emollients and thickeners in cosmetics and food industry.
Fatty alcohols are a common component of waxes, mostly as esters with fatty acids but also as alcohols themselves.
Nutrition
Very long chain fatty alcohols (VLCFA), obtained from plant waxes and beeswax have been reported to lower plasma cholesterol in humans. They can be found in unrefined cereal grains, beeswax, and many plant-derived foods. Reports suggest that 5–20 mg per day of mixed C24–C34 alcohols, including octacosanol and triacontanol, lower low-density lipoprotein (LDL) cholesterol by 21%–29% and raise high-density lipoprotein cholesterol by 8%–15%. Wax esters are hydrolyzed by a bile salt–dependent pancreatic carboxyl esterase, releasing long chain alcohols and fatty acids that are absorbed in the gastrointestinal tract. Studies of fatty alcohol metabolism in fibroblasts suggest that very long chain fatty alcohols, fatty aldehydes, and fatty acids are reversibly inter-converted in a fatty alcohol cycle. The metabolism of these compounds is impaired in several inherited human peroxisomal disorders, including adrenoleukodystrophy and Sjögren-Larsson syndrome. Concentrations of VLCFA in blood plasma increase during fasting and when children are placed a ketogenic diet to suppress seizures.Read more.....
15.00 |
Sulfuric acid
Many proteins are made of sulfur-containing amino acids (such as cysteine and methionine) which produce sulfuric acid when metabolized by the body.
Contents
Occurrence
Pure (undiluted) sulfuric acid is not encountered on Earth, due to sulfuric acid's great affinity for water. Apart from that, sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation of sulfur dioxide in the presence of water - i.e., oxidation of sulfurous acid. Sulfur dioxide is the main byproduct produced when sulfur-containing fuels such as coal or oil are burned.
Sulfuric acid is formed naturally by the oxidation of sulfide minerals, such as iron sulfide. The resulting water can be highly acidic and is called Acid Mine Drainage (AMD). This acidic water is capable of dissolving metals present in sulfide ores, which results in brightly-colored, toxic streams. The oxidation of iron sulfide pyrite by molecular oxygen produces iron(II), or Fe2+:
2 FeS2 + 7 O2 + 2 H2O → 2 Fe2+ + 4 SO42− + 4 H+.
The Fe2+ can be further oxidized to Fe3+, according to:
4 Fe2+ + O2 + 4 H+ → 4 Fe3+ + 2 H2O,
and the Fe3+ produced can be precipitated as the hydroxide or hydrous oxide. The equation for the formation of the hydroxide is
Fe3+ + 3 H2O → Fe(OH)3 + 3 H+.
The iron(III) ion ("ferric iron", in casual nomenclature) can also oxidize pyrite. When iron(III) oxidation of pyrite occurs, the process can become rapid. pH values below zero have been measured in ARD produced by this process.
ARD can also produce sulfuric acid at a slower rate, so that the Acid Neutralization Capacity (ANC) of the aquifer can neutralize the produced acid. In such cases, the Total Dissolved solids (TDS) concentration of the water can be increased form the dissolution of minerals from the acid-neutralization reaction with the minerals.
[edit] Extraterrestrial sulfuric acid
[edit] The cycle, in atmosphere of Venus
Sulfuric acid is produced in the upper atmosphere of Venus by the Sun's photochemical action on carbon dioxide, sulfur dioxide, and water vapor. Ultraviolet photons of wavelengths less than 169 nm can photodissociate carbon dioxide into carbon monoxide and atomic oxygen.
Atomic oxygen is highly reactive. When it reacts with sulfur dioxide, a trace component of the Venusian atmosphere, the result is sulfur trioxide, which can combine with water vapor, another trace component of Venus's atmosphere, to yield sulfuric acid.
CO2 → CO + O
SO2 + O → SO3
SO3 + H2O → H2SO4
In the upper, cooler portions of Venus's atmosphere, sulfuric acid exists as a liquid, and thick sulfuric acid clouds completely obscure the planet's surface when viewed from above. The main cloud layer extends from 45–70 km above the planet's surface, with thinner hazes extending as low as 30 and as high as 90 km above the surface.
The permanent venusian clouds produce a concentrated acid rain, as the clouds on the atmosphere of Earth produce water rains.
Thus, it's exist a double combined cycle of sulfur dioxide and water, because when sulfuric drops fall down, they are heated up and release water vapor, becoming more and more concentrated. And when they reach above 300°C, sulfuric acid begins to decompose in sulfur trioxide and water (both gaseous). sulfur trioxide is highly reactive (like sulfuric acid) and become sulfuric dioxide and oxygen, which oxides traces of CO, or surface rocks.
Sulfuric dioxide and water (vapor) continuously equilibrate their pressure from deep venusian atmosphere to upper altitudes, where they will be transformed again in sulfuric acid, and the cycle is closed !
[edit] On Europa's icy surface
Infrared spectra from NASA's Galileo mission show distinct absorptions on Jupiter's moon Europa that have been attributed to one or more sulfuric acid hydrates. The interpretation of the spectra is somewhat controversial. Some planetary scientists prefer to assign the spectral features to the sulfate ion, perhaps as part of one or more minerals on Europa's surface.
15.00 |
Ester reactions
* Esters may undergo hydrolysis - the breakdown of an ester by water. This process can be catalyzed both by acids and bases. The base-catalyzed process is called saponification. The hydrolysis yields an alcohol and a carboxylic acid or its carboxylate salt.
* Esters also react if heated with primary or secondary amines, producing amides.
* Phenyl esters react to hydroxyarylketones in the Fries rearrangement.
* Di-esters such as diethyl malonate react as nucleophile with alkyl halides in the malonic ester synthesis after deprotonation.
* Specific esters are functionalized with an α-hydroxyl group in the Chan rearrangement.
* Esters are converted to isocyanates through intermediate hydroxamic acids in the Lossen rearrangement.
* Esters with β-hydrogen atoms can be converted to alkenes in ester pyrolysis.
15.00 |
Ester

Esters are a class of chemical compounds and functional groups. Esters consist of an inorganic or organic acid in which at least one -OH (hydroxyl) group is replaced by an -O-alkyl (alkoxy) group. Some acids that are commonly esterified are carboxylic acids, phosphoric acid, sulfuric acid, nitric acid, and boric acid. Volatile esters, particularly carboxylate esters, often have a pleasant smell and are found in perfumes, essential oils, and pheromones, and give many fruits their scent. Ethyl acetate and methyl acetate are important solvents; fatty acid esters form fat and lipids; phosphoesters form the backbone of DNA molecules; nitrate esters are known for their explosive properties (best known: nitroglycerin) and polyesters are important plastics. Cyclic esters are called lactones. The name "ester" is derived from the German Essig-Äther (literally: vinegar ether), an old name for ethyl acetate. Esters can be synthesized in a condensation reaction between an acid and an alcohol in a reaction known as esterification.Read more.....
15.00 |
Dehydrogenation
There are a variety of classes of dehydrogenations:
* Aromatization - Six-membered alicyclic rings can be aromatized in the presence of hydrogenation catalysts, the elements sulfur and selenium, or quinones (such as DDQ).
* Oxidation - The conversion of alcohols to ketones or aldehydes can be effected by metal catalysts such as copper chromite. In the Oppenauer oxidation, hydrogen is transferred from one alcohol to another to bring about the oxidation.
* Dehydrogenation of amines - amines can be converted to nitriles using a variety of reagents, such as IF5.
* Dehydrogenation of paraffins and olefins - paraffins like n-pentane and isopentane can be converted to pentene and isoprene.
Dehydrogenation converts saturated fats to unsaturated fats.
Enzymes that catalyze dehydrogenation are called dehydrogenases.
15.00 |
Equipment for crystallization
2. Scraped surface crystallizers. One type of scraped surface crystallizer is the Swenson-Walker crystallizer, which consists of an open trough 0.6m wide with a semicircular bottom having a cooling jacket outside. A slow-speed spiral agitator rotates and suspends the growing crystals on turning. The blades pass close to the wall and break off any deposits of crystals on the cooled wall. The product generally has a somewhat wide crystal-size distribution.
3. Double-pipe scraped surface crystallizer. Also called a votator, this type of crystallizer is used in crystallizing ice cream and plasticizing margarine. Cooling water passes in the annular space. An internal agitator is fitted with spring-loaded scrapers that wipe the wall and provide good heat-transfer coefficients.
4. Circulating-liquid evaporator-crystallizer. Also called Oslo crystallizer. Here supersaturation is reached by evaporation. The circulating liquid is drawn by the screw pump down inside the tube side of the condensing stream heater. The heated liquid then flows into the vapor space, where flash evaporation occurs, giving some supersaturation.The vapor leaving is condensed. The supersaturated liquid flows down the downflow tube and then up through the bed of fluidized and agitated crystals, which are growing in size. The leaving saturated liquid then goes back as a recycle stream to the heater, where it is joined by the entering fluid. The larger crystals settle out and slurry of crystals and mother liquid is withdrawn as a product.
5. Circulating-magma vacuum crystallizer. The magma or suspension of crystals is circulated out of the main body through a circulating pipe by a screw pump. The magma flows though a heater, where its temperature is raised 2-6 K. The heated liquor then mixes with body slurry and boiling occurs at the liquid surface. This causes supersaturation in the swirling liquid near the surface, which deposits in the swirling suspended crystals until they leave again via the circulating pipe. The vapors leave through the top. A steam-jet ejector provides vacuum.
6. Continuous oscillatory baffled crystallizer (COBCTM). The COBCTM is a tubular baffled crystallizer that offers plug flow under laminar flow conditions (low flow rates) with superior heat transfer coefficient, allowing controlled cooling profiles, e.g. linear, parobolic, discontinued, step-wise or any type, to be achieved. This gives much better control over crystal size, morphology and consistent crystal products. For further information see oscillatory baffled reactor.
15.00 |
Crystal production

From a material industry perspective:
* Macroscopic crystal production, for supply the demand of natural-like crystals with methods that "accelerate time-scale" for massive production and/or perfection:
o ionic crystal production;
o covalent crystal production.
* Tiny size crystals:
o Powder, sand and smaller sizes: using methods for powder and controlled (nanotechnology fruits) forms.
+ Mass-production: on chemical industry, like salt-powder production.
+ Sample production: small production of tiny crystals for material characterization. Controlled recrystallization is an important method to supply unusual crystals, that are needed to reveal the molecular structure and nuclear forces inside a typical molecule of a crystal. Many techniques, like X-ray crystallography and NMR spectroscopy, are widely used in chemistry and biochemistry to determine the structures of an immense variety of molecules, including inorganic compounds and bio-macromolecules.
o Thin film production.
Massive production examples:
* "Powder salt for food" industry;
* Silicon crystal wafer production.
* Production of sucrose from sugar beet, where the sucrose is crystallized out from an aqueous solution.Read more.....
15.00 |
Ethyl acetate Uses
Ethyl acetate is primarily used as a solvent. For example, it is commonly used to clean circuit boards to wash away any remaining flux residue, to dissolve the pigments for nail varnishes, and is responsible for the solvent-effect of some nail varnish remover (acetone and acetonitrile are also used). Industrially it is used to decaffeinate coffee beans and tea leaves. It is also used in paints as an activator or hardener.
In the laboratory, mixtures of ethyl acetate and other solvents are commonly used in chromatography. It is also used as a solvent for extractions. Ethyl acetate is rarely selected as a reaction solvent because it is prone to hydrolysis.
Like most simple esters, ethyl acetate has a fruity smell. Ethyl acetate is present in confectionery, perfumes, and fruits. In perfumes, it evaporates quickly, leaving but the scent of the perfume on the skin.
• Occurrence in wines
Ethyl acetate is the most common ester found in wine, being the product of the most common volatile organic acid-acetic acid and the ethanol alcohol created during the fermentation of wine. The aroma of ethyl acetate is most vivid in younger wines and contribute towards the general perception of "fruitiness" in the wine. Sensitivity varies with most people having a perception threshold around 120 mg/l. Excessive amounts of ethyl acetate is considered a wine fault. Exposure to oxygen can exacerbate the fault due to the oxidation of ethanol creating acetaldehyde. This can leave the wine with a sharp vinegar like taste.
• Other uses
In the field of entomology, ethyl acetate is an effective poison for use in insect collecting and study. In a killing jar charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not hygroscopic, ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection.
15.00 |
Industrial production of Ethyl acetate
Catalysts for dehydrogenation include copper, operating at an elevated temperature but below 250 °C. The copper may have its surface area increased by depositing it on zinc, promoting the growth of snowflake, fractal like, structures. This surface area can be again increased by deposition onto a zeolite, typically ZSM-5. Traces of rare earth metals or alkalies, such as that of sodium and potassium, have also been found to be beneficial to the process. Byproducts of hydrogenation include diethyl ether (thought to primarily arise due to aluminum sites in the catalyst), acetaldehyde, acetaldehyde aldol products, higher esters and ketones. Acetaldehyde and MEK complicate conversion and purification as ethanol forms an azeotrope with water, as does ethyl acetate with ethanol and water and MEK with both ethanol and the acetate. To obtain a high purity product, these azeotropes must be "broken", and this can be achieved by making use of pressure swing distillation.
The composition of the distillate removed from the conversion products is biased towards acetate at atmospheric pressure and ethanol at increased pressure. First, the raw product is fed into a high pressure column where the bulk of the contaminating ethanol is removed. By then feeding the ethanol depleted distillate into a low pressure column, the acetate can be removed from the remaining ethanol azeotrope.
MEK forms during the conversion process from 2-butanol. The latter fails to form an azeotrope with the acetate and so MEK can be removed by hydrogenation of the contaminated product over nickel and further distillation to strip away 2-butanol. This provides the simultaneous benefit of removing the acetylaldehyde contaminant by returning it to an ethanol form and is easily accomplished as hydrogen is a byproduct of the initial dehydrogenation process.
It may also be possible to break the azeotropes with the use of membrane distillation, molecular sieves, an entrainer or absorption medium.
The distilled ethanol and rehydrogenated contaminants can then be recycled into the raw feedstock.
15.00 |
Thermodynamic view of crystallization
 The nature of a crystallization process is governed by both thermodynamic and kinetic factors, which can make it highly variable and difficult to control. Factors such as impurity level, mixing regime, vessel design, and cooling profile can have a major impact on the size, number, and shape of crystals produced.
The nature of a crystallization process is governed by both thermodynamic and kinetic factors, which can make it highly variable and difficult to control. Factors such as impurity level, mixing regime, vessel design, and cooling profile can have a major impact on the size, number, and shape of crystals produced.
Now put yourself in the place of a molecule within a pure and perfect crystal, being heated by an external source. At some sharply defined temperature, a bell rings, you must leave your neighbours, and the complicated architecture of the crystal collapses to that of a liquid. Textbook thermodynamics says that melting occurs because the entropy, S, gain in your system by spatial randomization of the molecules has overcome the enthalpy, H, loss due to breaking the crystal packing forces:
T(S{liquid} - S{solid}) > H{liquid} - H{solid}
G{liquid} <>
15.00 |
Purification of Crystallization

Well formed crystals are expected to be pure because each molecule or ion must fit perfectly into the lattice as it leaves the solution. Impurities would normally not fit as well in the lattice, and thus remain in solution preferentially. Hence, molecular recognition is the principle of purification in crystallization. However, there are instances when impurities incorporate into the lattice, hence, decreasing the level of purity of the final crystal product. Also, in some cases, the solvent may incorporate into the lattice forming a solvate. In addition, the solvent may be 'trapped' (in liquid state) within the crystal formed, and this phenomenon is known as inclusion.Read more.....
15.00 |
Crystallization
The crystallization process consists of two major events, nucleation and crystal growth. Nucleation is the step where the solute molecules dispersed in the solvent start to gather into clusters, on the nanometer scale (elevating solute concentration in a small region), that becomes stable under the current operating conditions. These stable clusters constitute the nuclei. However when the clusters are not stable, they redissolve. Therefore, the clusters need to reach a critical size in order to become stable nuclei. Such critical size is dictated by the operating conditions (temperature, supersaturation, etc.). It is at the stage of nucleation that the atoms arrange in a defined and periodic manner that defines the crystal structure — note that "crystal structure" is a special term that refers to the relative arrangement of the atoms, not the macroscopic properties of the crystal (size and shape), although those are a result of the internal crystal structure.
The crystal growth is the subsequent growth of the nuclei that succeed in achieving the critical cluster size. Nucleation and growth continue to occur simultaneously while the supersaturation exists. Supersaturation is the driving force of the crystallization, hence the rate of nucleation and growth is driven by the existing supersaturation in the solution. Depending upon the conditions, either nucleation or growth may be predominant over the other, and as a result, crystals with different sizes and shapes are obtained (control of crystal size and shape constitutes one of the main challenges in industrial manufacturing, such as for pharmaceuticals). Once the supersaturation is exhausted, the solid-liquid system reaches equilibrium and the crystallization is complete, unless the operating conditions are modified from equilibrium so as to supersaturate the solution again.
Many compounds have the ability to crystallize with different crystal structures, a phenomenon called polymorphism. Each polymorph is in fact a different thermodynamic solid state and crystal polymorphs of the same compound exhibit different physical properties, such as dissolution rate, shape (angles between facets and facet growth rates), melting point, etc. For this reason, polymorphism is of major importance in industrial manufacture of crystalline products.
There are many examples of natural process that involve crystallization.
Geological time scale process examples include:
* Natural (mineral) crystal formation (see also gemstone);
* Stalactite/stalagmite, rings formation.
Usual time scale process examples include:
* Snow flakes formation (see also Koch snowflake);
* Honey crystallization (nearly all types of honey crystallize).
15.00 |
Basic Steps in the Production of Ethyl Alcohol (part 2)
Instead of using commercial enzymes, it is possible to affect conversion by employing barley malt -- at the ratio of 15% by weight, or 7 pounds per bushel -- in both the pre- and post-boil. However, such a technique requires a more acidic medium (about pH 4-5) and lower temperatures -- about 145 deg F (63 deg C) is optimum -- than MOTHER's powders. Though the weights and temperatures differ, the same sequence is followed as discussed in "Conversion With MOTHER's Enzymes".
(One way to speed up the cooking process is with steam, which -- at 350 deg F, 177 deg C -- reduces the cooking time to one minute. Another commercial approach is to use extruders: machines much like meat grinders that compress, grind, and convert the grain in a one-step process.)
FERMENTATION
If you use barley malt for the conversion process -- or if you are following some alternative recipe that does not employ MOTHER's Fermentation Powder -- you will need to add your own yeast.
Mix up two ounces of distiller's or baker's yeast in a quart or two of the liquid mash, and add the concoction to the wort. Vigorous agitation will oxygenate the mixture and encourage a rapid initial growth of the yeast culture.
Yeast plants can propagate in a solution with or without air, so agitate only enough to saturate the wort with air and then let it stand still. If the mash is continually agitated, the yeast will reproduce faster and make less waste: carbon dioxide and alcohol. But if the solution becomes anaerobic (without air) the yeast slows down reproduction and makes more alcohol and carbon dioxide.
Yeast also produces enzymes of its own to convert complex sugars. Since sugar conversion and alcohol conversion can take place simultaneously, the amylase enzymes and the yeast work in cooperation to convert the dextrins to glucose and fructose and then to alcohol and C02.
Fermentation is a chemical process and produces heat. In concentrated or particularly large mashes, the temperature can actually rise to levels dangerous to yeast. Since the ideal temperature for yeast is around 85 deg F, it's best to maintain that temperature by either utilizing cooling coils or keeping the water-to-grain ratio at about 40 gallons per bushel.
Conversion of sugars to alcohol and C02 will be completed in three to five days, depending on the temperature of the mixture and the type of yeast used. You can tell when the mash is done by watching the "cap" of solids on top of the solution. During fermentation, the rising C02 keeps the solids in constant motion, but when the bubbling stops, the solids fall to the bottom. At this time, you're ready to separate the solids from the liquids and begin distillation.
KEEP IT CLEAN!
Remember, sanitation is extremely important! There are many kinds of invading bacteria, including strains which can withstand boiling temperatures. So, observe the same standards that any restaurant or kitchen follows. And keep the fermenting vat well covered: a fly in the ointment will turn your mash into something that it's best to keep upwind of.
15.00 |
Basic Steps in the Production of Ethyl Alcohol (part 1)
It is possible, however, to make alcohol from sugar-producing plants (saccharine material) such as sugar beets, sugarcane, fruits, and others. These substances need no milling (as do grains), but they do require some kind of grinding or squeezing process. Rapid, efficient fermentation of these sugars has not been as well explored as the process using starch.
A third source of fermentables is cellulose, as found in wood and waste sulphite liquor. This more complex process requires the use of acids to reduce the material to wood sugars. Consequently, most do-it-yourselfers should stick to either starch or sugar.
MILLING
All grains must be ground before mashing to expose the starch granules and help them remain in suspension in a water solution. The grain should be ground into a meal -- not a flour! -- that will pass a 20-mesh screen. On a hammermill, however, a 3/16" screen will suffice.
Potatoes and similar high-moisture starch crops should be sliced or finely chopped. Since potato starch granules are large and easily ruptured, it isn't necessary to maintain the hard rapid boil which is required of the tougher, dryer "flinty" starches found in grains.
CONVERSION WITH MOTHER'S ENZYMES
For small batches (5 bushels or less), fill the cooker with water (30 gallons per bushel), and add the meal slowly, to prevent lumps from forming. (When, cooking with steam, or at higher temperatures, it is possible to save energy by using less water at the beginning. But for the "small batcher" with an ordinary cooking apparatus, the most complete conversion is obtained by using the full amount of water right from the start to encourage a rapid rolling boil.)
Next, add 3 measuring spoons -- as provided -- per bushel of MOTHER's Alcohol Fuel Mash Cooking Enzyme (mixed in water) to the mixture and raise the temperature of the mash to 170 deg F (77 deg C), the optimum working environment for the enzyme. Hold the solution at that temperature for 15 minutes while agitating it vigorously.
At this point all the starch available at 170 deg F has been converted to dextrins, so it's time to raise the temperature of the mash to the boiling point. The concoction should be liquid enough to roll at its own rate -- if not, add 2 to 3 gallons of water. Hold the boil for 30 minutes to complete the liquefaction stage. All the starches are now in solution.
Now reduce the temperature to 170 deg F, using the cooling coil, and add 3 more measuring spoons per bushel of MOTHER's Cooking Enzyme (mixed in water). After 30 minutes of agitation at this temperature, all the previously released starches will have been reduced to dextrins, thereby completing primary conversion.
During secondary conversion the dextrins are further reduced to simple sugars (maltose and glucose) by the beta, or -- to be more exact -- glucoamylase enzymes. Because MOTHER's Alcohol Fuel Fermentation Powder contains both the enzymes and the yeast necessary to carry out secondary conversion and proper fermentation simultaneously, you can add 6 measuring spoons per bushel of the fermentation powder (mixed in water) as soon as you've brought the temperature down to 85 deg F (29 deg C) using the cooling coils.
15.00 |
Quinone
Orthobenzoquinone is the oxidized form of catechol (1,2-dihydroxybenzene), while parabenzoquinone is the oxidized form of hydroquinone. An acidic potassium iodide solution reduces a solution of benzoquinone to hydroquinone, which is oxidized back with a solution of silver nitrate.
Quinone is also the name for the class of compounds containing either benzoquinone isomers as part of their structure. Quinones are not aromatic, but are diketones.
Quinone is a common constituent of biologically relevant molecules (e.g. Vitamin K1 is phylloquinone). Others serve as electron acceptors in electron transport chains such as those in Photosystems I & II of photosynthesis, and aerobic respiration. A natural example of the oxidization of hydroquinone to quinone is the spray of bombardier beetles. Hydroquinone is reacted with hydrogen peroxide to produce a fiery blast of steam, a strong deterrent in the animal world. Quinones can be partially reduced to quinols.
Benzoquinone is used in organic chemistry as an oxidizing agent. Stronger quinone oxidising agents exist; for instance: 2,3,5,6-tetrachloro-parabenzoquinone (also known as p-chloranil) and 2,3-dicyano-5,6-dichloro-parabenzoquinone (also known as DDQ).
15.00 |
Esterification
Examples
• Heating to reflux an acid (usually, but not always a carboxylic acid) and a primary or secondary alcohol in the presence of a catalyst (commonly H2SO4) forms the ester, with water as a byproduct which can be removed to force the equilibrium in the desired direction. This method is called Fischer esterification. For example, esterification of acetic acid in excess ethanol (possibly as the solvent) in the presence of concentrated sulfuric acid as a catalyst results in an ester (ethyl acetate).
H3C-COOH + HO-CH2-CH3 → H3C-COO-CH2-CH3 + H2O (with the presence of conc. sulfuric acid)
• The reaction of an alkali carboxylate and an alkyl halide. This is not a reversible reaction and therefore can run to completion naturally. In the case that an alkyl chloride is used, iodide may be added to catalyze the reaction by a halide exchange mechanism. The carboxylate salt may be generated in situ or prior to the reaction. In difficult cases, the silver carboxylate may be used, since the silver ion coordinates to the halide aiding its departure and improving the reaction rate. This reaction can suffer from anion availability problems and therefore can benefit from the addition of phase transfer catalysts or highly polar aprotic solvents such as DMF. As an example, the reaction of sodium acetate with ethyl bromide is shown.
H3C-COO- Na+ + Br-CH2-CH3 → H3C-COO-CH2-CH3 + Br- + Na+
• The reaction of a carboxylic acid halogenide (which is also called acyl halide) with an alcohol/phenol. This reaction is usually very rapid due to the high reactivity of the acyl halide (it is often performed at low temperatures), but for the same reason it tends to be difficult to control, often resulting in a mixture of low purity products and a high percentage of by-products.
H3C-COCl + HO-CH2-CH3 → H3C-COO-CH2-CH3 + H-Cl
• The reaction of a carboxylic acid anhydride with an alcohol. This method is favored for the synthesis of phenyl esters (for example, it is used in the synthesis of aspirin). The anhydride may be generated in situ, and catalysts are usually added (often stoichiometric quantities of amines such as pyridine or triethylamine, which also serve to neutralize the acid formed). This method is very inefficient with respect to the acid (essentially 2 moles are required for each mole of alcohol), so is mainly used either for low molecular weight acids or for very expensive alcohols.
H3C-CO-O-CO-CH3 + HO-CH2-CH3 → H3C-COO-CH2-CH3 + H3C-COOH
15.00 |
Equipment used for hydrogenation
* Batch hydrogenation under atmospheric conditions
* Batch hydrogenation at elevated temperature and/or pressure
* Flow hydrogenation
• Batch hydrogenation under atmospheric conditions
The original and still the most commonly practised form of hydrogenation, this process is usually effected by adding solid catalyst to a round bottom flask of dissolved reactant which has been evacuated using nitrogen or argon gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then applied by fixing a balloon filled from a cylinder to a syringe and needle using laboratory tape and inserting the needle through the rubber seal, with the resulting three phase mixture being mechanically stirred until the reaction has gone to completion.
Some scientists prefer to measure hydrogen uptake to monitor the process of their reaction. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate, or investing in a hydrogenation laboratory equipped with gauges for each reaction vessel.
• Batch hydrogenation at elevated temperature and/or pressure
Many key hydrogenation reactions such as hydrogenolysis of protecting groups and the reduction of aromatic systems proceed extremely sluggishly (if at all) at atmospheric temperature and pressure, leading to the popularity of pressurised systems. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a pressure vessel. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source and the system is mechanically rocked to provide agitation. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility. This vastly increases the rate of reaction as described by the Arrhenius equation.
• Flow Hydrogenation
In recent times, flow hydrogenation has become a very popular technique at the bench and increasingly the process scale. This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen. Using established HPLC technology, this technique allows the application of pressures from atmospheric to 1,450 PSI. Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering pyrophoric catalysts.
21.45 |
Hydrogenation in the food industry
Hydrogenation results in the conversion of liquid vegetable oils to solid or semi-solid fats, such as those present in margarine. Changing the degree of saturation of the fat changes some important physical properties such as the melting point, which is why liquid oils become semi-solid. Semi-solid fats are preferred for baking because the way the fat mixes with flour produces a more desirable texture in the baked product. Since partially hydrogenated vegetable oils are cheaper than animal source fats, are available in a wide range of consistencies, and have other desirable characteristics (e.g., increased oxidative stability (longer shelf life)), they are the predominant fats used in most commercial baked goods. Fat blends formulated for this purpose are called shortenings.
• Health implications
A side effect of incomplete hydrogenation having implications for human health is the isomerization of the remaining unsaturated carbon bonds. The cis configuration of these double bonds predominates in the unprocessed fats in most edible fat sources, but incomplete hydrogenation partially converts these molecules to trans isomers, which have been implicated in circulatory diseases including heart disease (see trans fats). The catalytic hydrogenation process favors the conversion from cis to trans bonds because the trans configuration has lower energy than the natural cis one. At equilibrium, the trans/cis isomer ratio is about 2:1. Food legislation in the US and codes of practice in EU has long required labels declaring the fat content of foods in retail trade, and more recently, have also required declaration of the trans fat content. Further, trans fats are banned in Denmark, Switzerland, and New York City.
15.00 |
Thermodynamics and mechanism of hydrogenation
RCH=CH2 + D2 → RCHDCH2D
• Heterogeneous catalysis
On solids, the accepted mechanism today is called the Horiuti-Polanyi mechanism.
1. Binding of the unsaturated bond, and hydrogen dissociation into atomic hydrogen onto the catalyst
2. Addition of one atom of hydrogen; this step is reversible
3. Addition of the second atom; effectively irreversible under hydrogenating conditions
• Homogeneous catalysis
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal(H)2 complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps:
* binding of the hydrogen to give a dihydride complex ("oxidative addition"):
LnM + H2 → LnMH2
* binding of alkene:
LnM(η2H2) + CH2=CHR → Ln-1MH2(CH2=CHR) + L
* transfer of one hydrogen atom from the metal to carbon (migratory insertion)
Ln-1MH2(CH2=CHR) → Ln-1M(H)(CH2-CH2R)
* transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
Ln-1M(H)(CH2-CH2R) → Ln-1M + CH3-CH2R
Preceding the oxidative addition of H2 is the formation of a dihydrogen complex.
15.00 |
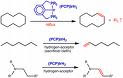
Hydrogenation
Because of the importance of hydrogen, many related reactions have been developed for its use. Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative sources of hydrogen, not H2: these processes are called transfer hydrogenations. The reverse reaction, removal of hydrogen from a molecule, is called dehydrogenation. A reaction where bonds are broken while hydrogen is added is called hydrogenolysis, a reaction that may occur to carbon-carbon and carbon-heteroatom (O, N, X) bonds. Hydrogenation differs from protonation or hydride addition: in hydrogenation, the products have the same charge as the reactants.
An illustrative example of a hydrogenation reaction is the addition of hydrogen to maleic acid to succinic acid depicted on the right. Numerous important applications are found in the petrochemical, pharmaceutical and food industries. Hydrogenation of unsaturated fats produces saturated fats and, in some cases, trans fats.
15.00 |